Introducing the Colligative Properties Worksheet with Answers, an invaluable resource for understanding the fundamental principles of colligative properties. This worksheet provides a comprehensive overview of the topic, including detailed explanations, formulas, and solved examples.
Colligative properties are solution properties that depend solely on the concentration of solute particles, regardless of their identity. These properties play a crucial role in various scientific fields, including chemistry, biology, and medicine.
Introduction to Colligative Properties
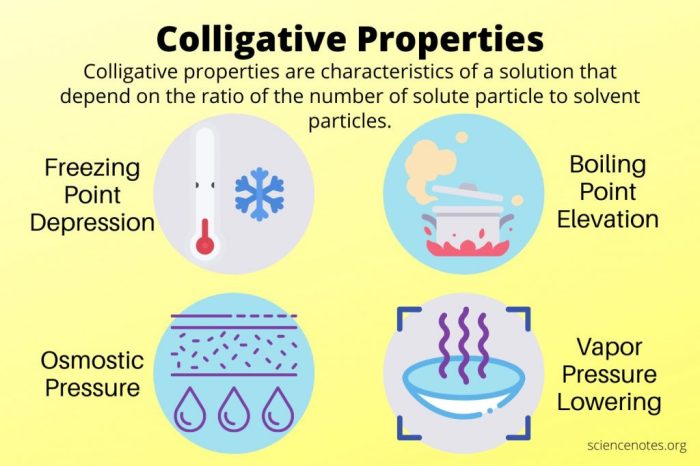
Colligative properties are physical properties of solutions that depend on the concentration of solute particles, not their identity. They include boiling point elevation, freezing point depression, and osmotic pressure.
Boiling Point Elevation
Boiling point elevation is the increase in the boiling point of a solution compared to the boiling point of the pure solvent. It is directly proportional to the molality of the solution.
Formula: ΔTb = Kb – m
Where:
- ΔTb is the boiling point elevation
- Kb is the boiling point elevation constant of the solvent
- m is the molality of the solution
Factors affecting boiling point elevation include solute concentration and solvent properties.
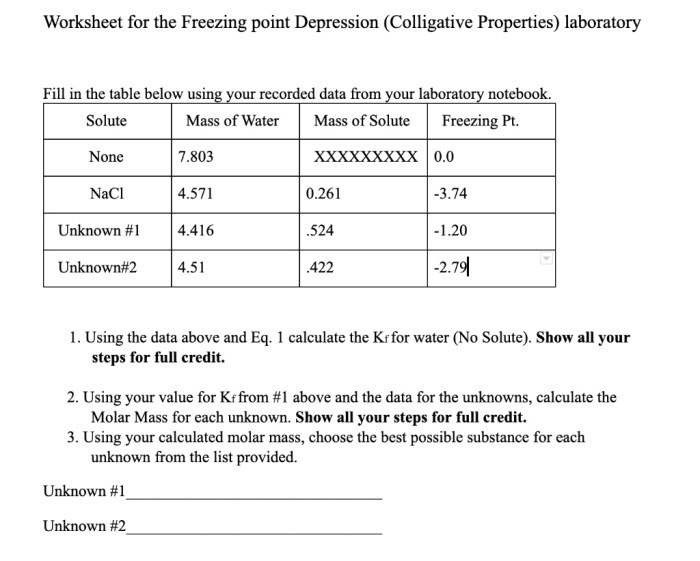
Freezing Point Depression, Colligative properties worksheet with answers
Freezing point depression is the decrease in the freezing point of a solution compared to the freezing point of the pure solvent. It is also directly proportional to the molality of the solution.
Formula: ΔTf = Kf – m
Where:
- ΔTf is the freezing point depression
- Kf is the freezing point depression constant of the solvent
- m is the molality of the solution
Factors affecting freezing point depression include solute concentration and solvent properties.
Osmotic Pressure
Osmotic pressure is the pressure that must be applied to a solution to prevent the flow of water across a semipermeable membrane. It is directly proportional to the concentration of solute particles in the solution.
Formula: Π = M – R – T
Where:
- Π is the osmotic pressure
- M is the molarity of the solution
- R is the ideal gas constant
- T is the temperature in Kelvin
Factors affecting osmotic pressure include solute concentration, temperature, and membrane permeability.
Applications of Colligative Properties
Colligative properties have various applications in chemistry, biology, and medicine.
- Determination of molecular weights
- Prediction of behavior of solutions
- Design of experiments
Detailed FAQs: Colligative Properties Worksheet With Answers
What are colligative properties?
Colligative properties are solution properties that depend only on the concentration of solute particles, not their identity.
What are some examples of colligative properties?
Boiling point elevation, freezing point depression, and osmotic pressure are common examples of colligative properties.
How can I use the Colligative Properties Worksheet with Answers?
The worksheet provides step-by-step guidance, formulas, and solved examples to help you understand and apply colligative properties in practice.