Molecular and empirical formula worksheet with answers – Embark on an enlightening journey into the realm of molecular and empirical formulas with our comprehensive worksheet and answer key. This invaluable resource empowers you to decipher the intricate language of chemical compounds, unraveling their composition and unlocking a deeper understanding of their properties and behavior.
Throughout this guide, we will delve into the intricacies of molecular and empirical formulas, equipping you with the knowledge and skills to navigate the complexities of chemical calculations with precision and confidence.
1. Introduction to Molecular and Empirical Formulas
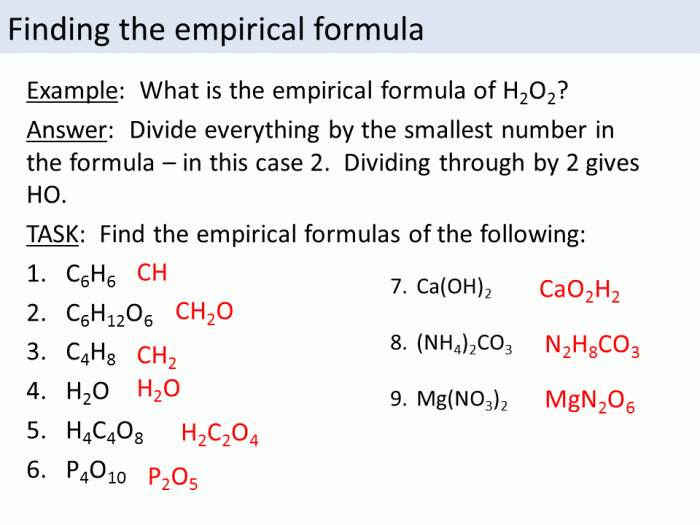
Molecular and empirical formulas are two different ways of representing the composition of a compound. A molecular formula shows the exact number of atoms of each element in a molecule of a compound, while an empirical formula shows the simplest whole-number ratio of atoms of each element in a compound.
For example, the molecular formula of glucose is C 6H 12O 6, which means that each molecule of glucose contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. The empirical formula of glucose is CH 2O, which means that the compound contains one carbon atom for every two hydrogen atoms and one oxygen atom.
2. Determining Molecular and Empirical Formulas
Determining the Molecular Formula from the Empirical Formula
To determine the molecular formula from the empirical formula, you need to know the molar mass of the compound. Once you know the molar mass, you can divide the molar mass by the empirical formula mass to get the molecular formula.
For example, the empirical formula of a compound is CH 2O. The molar mass of the compound is 30 g/mol. To find the molecular formula, divide the molar mass by the empirical formula mass: 30 g/mol ÷ 30 g/mol = 1. This means that the molecular formula is the same as the empirical formula, CH 2O.
Determining the Empirical Formula from the Molecular Formula, Molecular and empirical formula worksheet with answers
To determine the empirical formula from the molecular formula, you need to divide the subscripts of each element in the molecular formula by the greatest common factor of the subscripts. The resulting numbers are the subscripts of the elements in the empirical formula.
For example, the molecular formula of a compound is C 6H 12O 6. The greatest common factor of the subscripts is 6. To find the empirical formula, divide each subscript by 6: 6 ÷ 6 = 1, 12 ÷ 6 = 2, 6 ÷ 6 = 1. This means that the empirical formula of the compound is CH 2O.
3. Using Molecular and Empirical Formulas in Calculations

Calculating Molar Mass
The molar mass of a compound is the mass of one mole of the compound. To calculate the molar mass of a compound, multiply the atomic mass of each element in the compound by the number of atoms of that element in the compound and then add the products together.
For example, the molecular formula of glucose is C 6H 12O 6. The atomic mass of carbon is 12.01 g/mol, the atomic mass of hydrogen is 1.008 g/mol, and the atomic mass of oxygen is 16.00 g/mol. To calculate the molar mass of glucose, multiply the atomic mass of each element by the number of atoms of that element in the compound and then add the products together: (6 × 12.01 g/mol) + (12 × 1.008 g/mol) + (6 × 16.00 g/mol) = 180.156 g/mol.
Calculating the Number of Moles of a Substance
The number of moles of a substance is the amount of the substance in moles. To calculate the number of moles of a substance, divide the mass of the substance by the molar mass of the substance.
For example, if you have 100 g of glucose, the number of moles of glucose is 100 g ÷ 180.156 g/mol = 0.555 mol.
Calculating the Mass of a Substance
The mass of a substance is the amount of the substance in grams. To calculate the mass of a substance, multiply the number of moles of the substance by the molar mass of the substance.
For example, if you have 0.555 mol of glucose, the mass of glucose is 0.555 mol × 180.156 g/mol = 100 g.
4. Practice Problems

| Problem | Answer |
|---|---|
| What is the molecular formula of a compound with an empirical formula of CH2O and a molar mass of 60 g/mol? | C2H4O2 |
| What is the empirical formula of a compound with a molecular formula of C6H12O6? | CH2O |
| What is the molar mass of a compound with a molecular formula of NaCl? | 58.44 g/mol |
| How many moles of glucose are in 100 g of glucose? | 0.555 mol |
5. Applications of Molecular and Empirical Formulas
Molecular and empirical formulas are used in a variety of applications in chemistry. Some of the most common applications include:
- Identifying compounds
- Calculating the molar mass of compounds
- Calculating the number of moles of a substance
- Calculating the mass of a substance
- Predicting the properties of compounds
Molecular and empirical formulas are essential tools for chemists. They are used in a wide variety of applications, from identifying compounds to predicting their properties.
FAQ: Molecular And Empirical Formula Worksheet With Answers
What is the difference between a molecular formula and an empirical formula?
A molecular formula represents the exact number and type of atoms in a molecule, while an empirical formula represents the simplest whole-number ratio of atoms in a compound.
How do I determine the molecular formula from the empirical formula?
To determine the molecular formula from the empirical formula, you need to know the molar mass of the compound and the empirical formula mass.
What are some applications of molecular and empirical formulas?
Molecular and empirical formulas are used in various fields of chemistry, including organic chemistry, inorganic chemistry, biochemistry, and analytical chemistry.